


左氧氟沙星是氧氟沙星的左旋体,其抗菌活性约为氧氟沙星的两倍。其为第三代氟喹诺酮类抗菌药物,在组织中分布广泛,抗菌效应长、生物利用度高,适用于革兰阴性菌、革兰阳性菌、压氧菌等引起的皮肤软组织、呼吸系统、消化系统、泌尿系统感染。
Packaging: 400盒/件
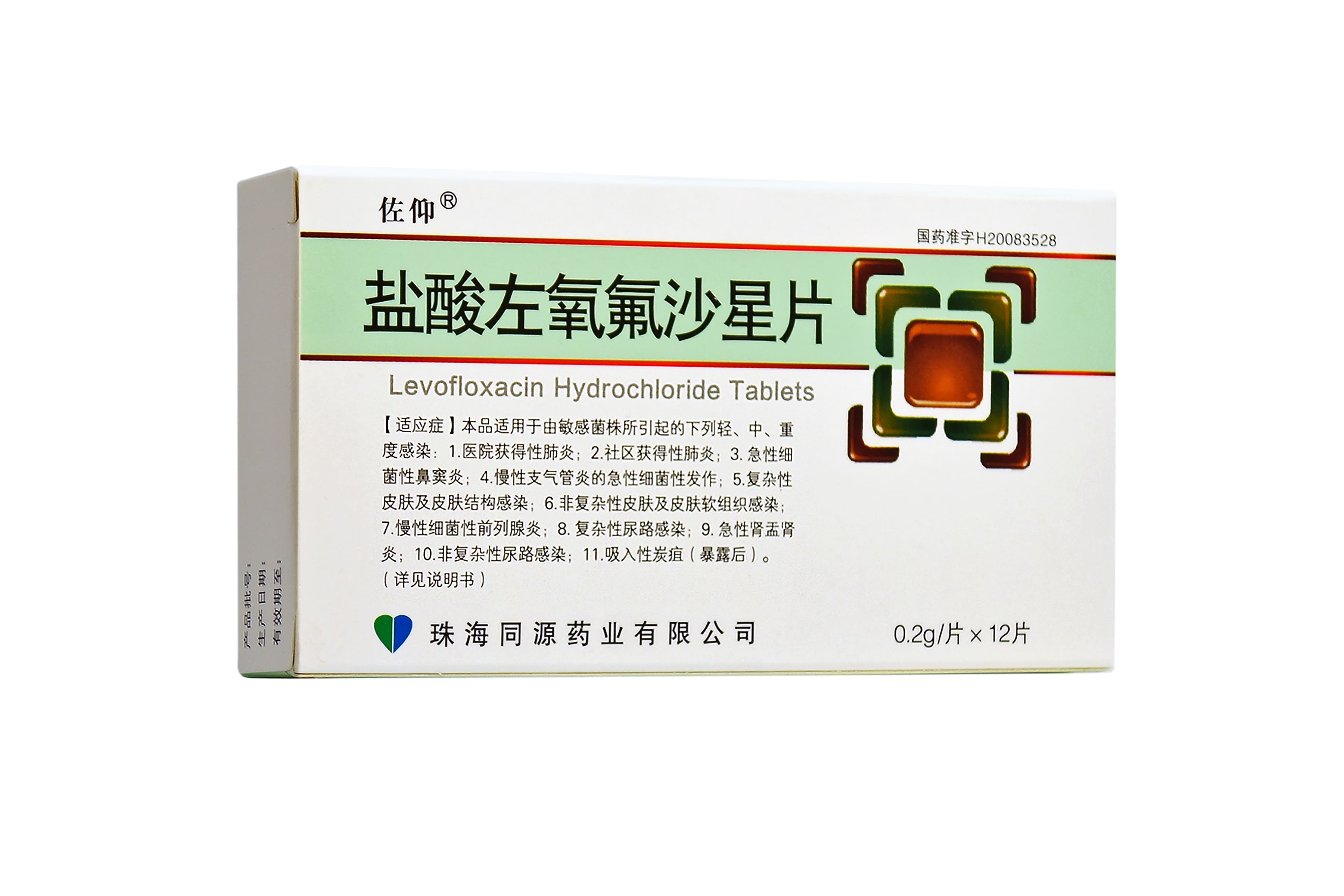
Specification: 0.2g*14片 0.2g*12片 0.2g*6片
Shelf Life: 24个月
Approval Number: 国药准字H20083528
Indications: 为减少耐药菌的产生,保证盐酸左氧氟沙星及其他抗菌药物的有效性,左氧氟沙星只用于治疗或预防已证明或高度怀疑由敏感细菌引起的感染。在选择或修改抗菌药物治疗方案时,应考虑细菌培养和药敏试验的结果。如果没有这些试验的数据做参考,则应根据当地流行病学和病原菌敏性进行经验性治疗。 在治疗前应进行细菌培养和药敏试验以分离并鉴定感染病原菌,确定其对盐酸左氧氟沙星的敏感性。在获得以上检验结果之前可以先使用左氧氟沙星进行治疗,得到检验结果之后再选择适当的治疗方法。 与此类中的其他药物相同,使用盐酸左氧氟沙星进行治疗时,铜绿假单胞菌的某些菌株可以很快产生耐药性。在治疗期间应定期进行细菌培养和药敏试验以掌握病原菌是否对抗菌药物持续敏感,并在细菌出现耐药性后能够及时发现。 盐酸左氧氟沙星口服制剂和注射剂可用于治疗成年人由下列细菌的敏感菌株所引起的下列轻、中、重度感染。 1.医院获得性肺炎;2.社区获得性肺炎;3.急性细菌性鼻窦炎;4.慢性支气管炎的急性细菌性发作;5.复杂性皮肤及皮肤结构感染;6.非复杂性皮肤及皮肤软组织感染;7.慢性细菌性前列腺炎;8.复杂性尿路感染;9.急性肾盂肾炎;10.非复杂性尿路感染;11.吸入性炭疽(暴露后)。